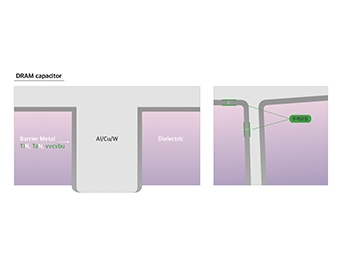
Barrier Metal (diffusion barrier material) is used to prevent contamination of the insulating layer caused by the diffusion of metal ions, oxygen, or moisture into the interlayer dielectric during metallization formation. As metallization metals like Al and Cu easily react with surrounding insulating layers, potentially compromising the reliability of semiconductor devices, the use of diffusion barriers becomes increasingly important as devices become finer.
Since the diffusion barriers must have anti-diffusion properties while passing electricity, Ti(N), Ta(N), Ru, etc. are used.
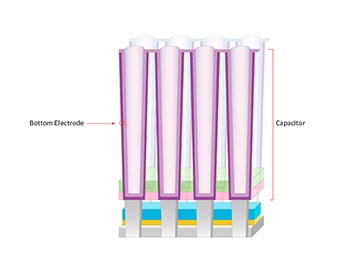
Electrode material is the electrode material of a capacitor that stores electrons in a memory device, and requires a structurally strong material with low contact resistance.
Until recently, TiN has been used as the bottom electrode. However, due to the miniaturization of line widths, materials such as Ru and Nb are being researched as potential alternatives.)
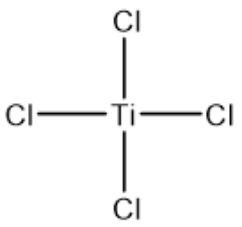
| Chemical name | Titanium(Ⅳ) chloride |
|---|---|
| Cas No. | 7550-45-0 |
| Molecular formula | TiCl4 |
| Molar Weight | 189.67 g/mol |
| Physical State / Color | Colorless liquid |
| Boiling point | 136.4 ℃ |
| Vapor pressure | 38℃ / 9.75 Torr |
| Water reactivity | Violently reacts |
